Shanghai Synology Research Institute has achieved important breakthrough in direct synthesis of syngas into olefins
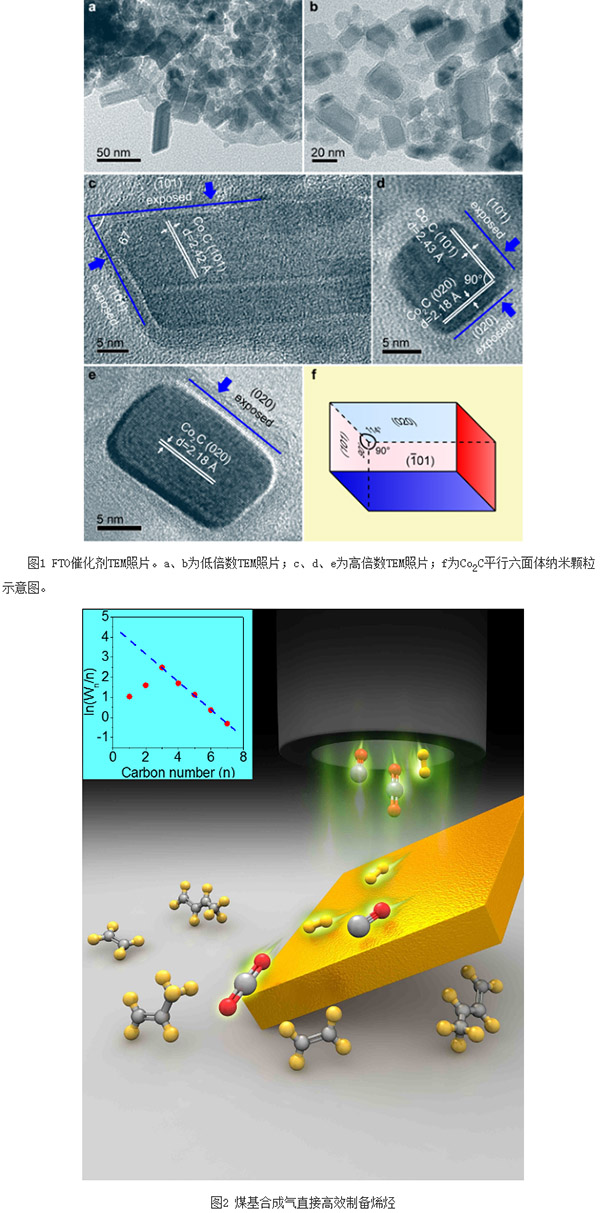
The joint research team of the Shanghai Institute of Advanced Study and the Shanghai University of Science and Technology of the Chinese Academy of Sciences has made significant progress in the direct synthesis of olefins from syngas, and Nature published its relevant results on October 6th. Among them, Shanghai University, East China Normal University, Institute of Physics of the Chinese Academy of Sciences and the Institute of Chemistry participated in part of the work. By adopting a new catalyst active-site structure, this research has realized the high selectivity and direct production of olefins under mild conditions, which is of great significance in expanding the field of syngas catalytic conversion. At the same time, the research results have high economic benefits and will help promote the development of China's coal chemical industry. This research work has received strong support from the National Natural Science Foundation of China, the Ministry of Science and Technology, the Shanghai Municipal Science and Technology Commission, Shanxi Luan Group and the Chinese Academy of Sciences. In the field of energy and chemical industry, olefins are a basic and very important high value-added chemical raw materials. Many products such as synthetic fibers, synthetic rubbers, synthetic plastics, advanced lubricating oils, high-carbon alcohols, and high-density jet fuels are based on them. raw material. Therefore, the level of development of the olefin industry and the balance of market supply and demand directly affect the development level and industry scale of the entire chemical industry. In recent years, to ease the reliance on petroleum resources, research at home and abroad has focused mainly on non-oil routes, that is, using coal or natural gas resources to directly or indirectly produce olefins. In the current mainstream process, firstly, the synthesis gas (main components are carbon monoxide and hydrogen, ie, CO and H2) is produced from coal or natural gas, and then the methanol from the synthesis gas is finally passed through the methanol conversion route (including methanol to ethylene, The MTO process for propylene and the MTP process for methanol to propylene) produces olefin products. The technology involves two major steps, namely synthesis gas synthesis of methanol via a copper-based catalyst, and conversion of methanol to olefins over a molecular sieve catalyst. Undoubtedly, if the reaction steps can be reduced, direct synthesis of olefins with high selectivity to syngas will show the advantages of a shorter process and lower energy consumption. The syngas-bearing reaction route directly produces olefins, which refers to the process of CO and H2 synthesizing olefins (also called FTO) through the Fischer-Tropsch (FT) reaction route under the action of a catalyst. In the FT synthesis reaction, it is generally believed that carbon-oxygen bond cleavage forms carbon-adsorbing intermediate species first, and then carbon-carbon bonds form products with different carbon chain lengths. For the classical FT mechanism, it is generally believed that the chain extension of the product obeys the polymerization mechanism, that is, the product selectivity approximates the distribution of Amderson-Schulz-Flory (ASF), and different values ​​of chain growth factor (α) correspond to different product distributions. At present, the main problem of FTO is the improvement of olefin selectivity and effective control of product distribution. Because FTO is a strongly exothermic reaction, excessively high reaction heat can easily cause local overheating, resulting in the occurrence of fly-temperature phenomenon, and the promotion of methanation and carbon deposition, especially due to the restrictions on the distribution of ASF and the limitations of kinetics and thermodynamics. The production of olefins severely reduces the total olefin yield. In addition, since olefins are an intermediate product in the FT synthesis process, secondary hydrogenation reactions can easily occur and be converted into saturated alkanes, thereby further reducing olefin selectivity. Since the direct synthesis of olefins from synthesis gas is constrained by the above factors, it is necessary to develop a new catalytically active bit structure in order to achieve good FTO catalytic performance, try to get rid of the limitations of ASF distribution, and reflect low methane selectivity and high olefin selectivity. . The Key Laboratory of Low Carbon Conversion Science and Engineering of the Shanghai Research Institute of the Chinese Academy of Sciences (Low Carbon Conversion Laboratory) is mainly engaged in the research and development of core technologies for low carbon conversion and utilization of carbon-containing resources. In order to provide technical support and solutions for the development of non-oil-dependent energy and chemical industries, low-carbon conversion laboratories have been devoted to the study of structure-activity relationships and reaction networks for syngas catalytic conversion and catalyst development. Recently, the Low Carbon Conversion Laboratory has creatively developed a brand-new catalyst and found that under mild reaction conditions (250 oC and 1 to 5 atm), the catalyst can achieve high selectivity of syngas directly to produce olefins, methane selectivity It can be as low as 5%, selectivity to light olefins up to 60%, selectivity to total olefins up to 80%, olefin/alkane ratio up to 30 or more; at the same time, the product carbon number exhibits a significant narrow-range high selectivity distribution, C2 The -15 selectivity accounts for more than 90%, and the product distribution completely disobeys the classic ASF rule, showing good FTO performance. In order to determine the nature of the active site, the laboratory, through in-depth structure-activity relationship studies and combined with DFT theoretical calculations, determined that the active site structure is a Co2C nanoparallel hexahedron with exposed surfaces of {101} and {020} (Figure 1). Co2C is generally regarded as one of the main reasons for the deactivation of Co-based FT catalysts, namely the low activity of Co2C and the high selectivity of CH4 during the synthesis gas conversion. This work reveals that there is a significant crystal surface effect in Co2C. Compared to other exposed surfaces, the {101} crystal plane is very favorable for the production of olefins, while the {101} and {020} crystal planes can effectively inhibit the formation of methane. Therefore, the Co2C nanoparallel hexahedrons with {101} and {020} exposed surfaces exhibited catalytic properties that were completely different from the traditional FT active phases. The methane selectivity was very low and the olefin selectivity was high. The products deviated from the classical ASF rule and reflected a narrow interval. Highly selective distribution. Based on the characteristics of China's lack of oil, low gas, and rich coal resources, the technology has a strong industrial application prospects and high economic benefits. In this scientific exploration and research, CASS Shanghai and Shanghai University of Science and Technology give full play to the advantages of the integration of science and education, especially in the areas of complementary talents, sharing of equipment and equipment platforms, and joint training of postgraduate students. The National Science Center has strategic opportunities and actively explores the effective implementation of the spillover effect and the radiative driving effect of the Center for Science and Technology Innovation. At the same time, under the strong support of the State and Shanghai, the Low-Carbon Laboratory has conducted in-depth cooperation with relevant universities, research institutes and resource-based state-owned enterprises in the process of optimizing the technological route, developing high-value-added products, and upgrading industrial energy levels. We have conducted basic research throughout, consolidating scientific issues, and working together to solve problems, and achieved very good results in opening up the innovation value chain. At present, the Shanghai Institute of High Technology of the Chinese Academy of Sciences has reached an agreement with the cooperative unit Shanxi Panan Group and other companies to jointly cooperate in the catalyst amplification preparation, reactor design and process development, and strive to achieve industrial demonstration and industrialization as soon as possible to promote China's coal Chemical development. Uvc Light,T5 Uvc Lamp,T5 Uvc Tube,T5 Uvc Bulb Changxing leboom lighting product CO.Ltd. , https://www.leboomuvd.com